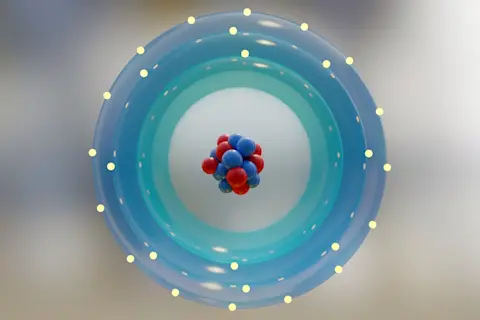
Atoms touch all the time! But to understand why we first have to decide what we mean by the word “touch.”
Our normal conception of touching is grounded in the macroscopic world. I put a cup on the table – the cup is touching the table. You dip your toes in the water – you are touching the water, and so on. In all these cases, one solid boundary or surface (the bottom of the cup, the edge of your toe) touches another solid boundary or surface (the top of the table, the surface of the ocean). But our macroscopic conceptions break down at the microscopic level, which is where the confusion over “touching” comes in.
Read More:Will Humans Ever Go Faster Than Light?
If we could zoom into atomic scales, we would see a madhouse. Atoms and molecules are constantly flying around, bumping into each other, twisting, spinning and ...