Molecules containing noble gases shouldn’t exist. By definition, these chemical elements — helium, neon, argon, krypton, xenon and radon — are the party poopers of the periodic table, huddling in the rightmost column and refusing to make molecules. Indeed, no one has ever seen any naturally occurring noble gas molecules on Earth. Earlier this decade, though, astronomers accidentally discovered one of these aloof elements in molecules in space.
Then, in 2019, observers reported finding a second kind of noble gas molecule, one they had sought for more than three decades and of a type that was the very first to form after the universe’s birth in the big bang. This newly found molecule lends insight into the chemistry of the early universe, before any stars began to shine or any galaxies had formed. The discovery may even help astronomers understand how the first stars arose.
Most chemical elements readily share electrons with other elements to make molecules, but noble gases normally don’t. “Noble gases are in some sense happy as they are,” says Peter Schilke, an astrophysicist at the University of Cologne in Germany. That’s because the outer shell of a noble gas atom already has its fill of electrons, so it won’t ordinarily exchange electrons to bond with other atoms and form molecules — at least, not here on Earth.
In retrospect, space seems the perfect place to seek noble gas molecules, because these gases abound in the cosmos. Helium is the second most common element in the universe, after hydrogen, and neon ranks fifth or sixth. And in interstellar space, where extreme temperatures and densities are the rule, noble gases do things they would never do on Earth. That includes forming molecules.
In addition to providing insight into the universe’s infancy, these exotic molecules tell scientists about the current conditions in the space between the stars — the gases that make up the interstellar medium — which is of intense interest to astronomers. “The interstellar medium is the place where stars and planetary systems are born,” says Maryvonne Gerin, an astrophysicist at the Observatory of Paris and coauthor of a 2016 Annual Review of Astronomy and Astrophysics article on interstellar molecules.
Earthly Noble Gas Discoveries
Here on Earth, scientists have been concocting noble gas molecules for nearly a century. In 1925, laboratory scientists were able to force the noble gas helium into a bond with hydrogen to form helium hydride, or HeH+ — termed a molecule by astronomers but, because it’s electrically charged, a molecular ion by chemists.
In 1962 chemist Neil Bartlett coaxed xenon to mate with fluorine and platinum, yielding a mustard-colored compound that was a first: a substance consisting of electrically neutral molecules which both astronomers and chemists are happy to say is full of noble gas molecules. Still, no one has ever seen any naturally occurring noble gas molecules on Earth.
For decades astronomers have pursued one noble gas molecule in particular: helium hydride, or HeH+, made of the two most common elements in the universe and thus a good bet to exist in space. Though naturally occurring helium hydride has never been found on Earth, scientists were able to force the two atoms together in the lab almost a century ago.
So it seemed this combo would be the most likely quarry for astronomers as well. Instead, they were caught off guard by an even stranger molecule.
An Interstellar Embarrassment
Argon is more than 20 times as common in Earth’s atmosphere as carbon dioxide but gets far less press. In fact, it is the third most abundant gas in the air you breathe. Nitrogen and oxygen make up 78 percent and 21 percent of Earth’s atmosphere, respectively, while argon accounts for most of the remaining 1 percent.
But nobody was looking for an interstellar molecule containing argon. “It was basically a serendipitous discovery,” says University College London astrophysicist Mike Barlow, who led the team that accidentally found ArH+: argonium, which consists of argon and hydrogen.
Noble gases (rightmost column, red) are known for being chemically non-reactive and don’t naturally bond with other atoms to form molecules on Earth. But it’s a different story in space. In the last decade, astronomers have discovered two examples of chemical compounds made of the noble gases helium and argon in space.
Another noble gas element helped to make the find possible. In 2009 the Herschel Space Observatory lifted off for space and literally kept its cool during the mission by carrying a tank of frigid liquid helium that lasted four years. This allowed Herschel to observe far-infrared wavelengths from distant objects without the interference its own warmth would have produced. Because many molecules absorb and emit far-infrared light, this spectral range is a good place to seek new space molecules.
Within a year of Herschel’s launch, astronomers began noticing that something in interstellar space was absorbing far-infrared light at a wavelength of 485 microns, a spectral line that hadn’t been observed before. “Nobody could figure out what it was,” says David Neufeld, an astrophysicist at Johns Hopkins University and coauthor of the 2016 Annual Review article (and an acquaintance of the author of this story in graduate school).
Schilke consulted colleagues in his group at Cologne and elsewhere. “We sat in the office at the whiteboard,” he says, “and we put all the possible molecules on there, including argonium.” No known molecule matched the observed wavelength of 485 microns.
Meanwhile, Barlow’s team was using Herschel data to study the Crab Nebula, the remains of a massive star our ancestors saw explode in the year 1054. The celestial fireworks forged argon and other “metals,” which astronomers define as all elements heavier than helium.

Another view of the Crab Nebula, the remnants of a supernova explosion witnessed by skywatchers in Japan and China a thousand years ago. Orange filaments reveal the hydrogen that once constituted the star; the blue glow is produced by the neutron star at the nebula’s center. Study of the light from the object revealed the presence of argonium. (Credit: NASA/ESA/J. Hester (Arizona State University))
NASA/ESA/J. Hester (Arizona State University
In the nebula’s argon-rich gas, Barlow and his colleagues spotted two unidentified spectral lines. One was the same mysterious line everyone else had been seeing at 485 microns; the other had exactly half the wavelength — the hallmark of a molecule containing two atoms. Barlow identified it as argonium, publishing the discovery in 2013. It was the first noble gas molecule ever found in nature. (Barlow notes that at the last minute the editors of his scientific paper changed “molecule” in the title to “molecular ion.”)
The discovery was a shock. “We were just stunned when we heard this,” Neufeld says. After all, astronomers had been seeing that same 485-micron spectral line elsewhere. “When I first heard about the detection,” Schilke says, “I was extremely embarrassed that we had not spotted this ourselves.”
The scientists were the victims of a down-to-earth mix-up. They thought they knew the wavelengths argonium produced, because scientists had created it in the lab decades earlier and measured its spectrum. But these laboratory molecules contained argon-40, which is by far the most common argon isotope — on Earth. But that’s only because the argon we breathe comes from the radioactive decay of potassium-40 in rocks.
The universe is different. “In the interstellar medium,” says Schilke, “argon-36 is by far the most abundant, and we were just too stupid to realize it.” Argonium made with argon-36 absorbs and emits light at slightly different wavelengths than it does with argon-40, explaining why the scientists had missed the identification.
Nevertheless, once they recognized the existence of interstellar argonium, Schilke, Neufeld, Gerin and their colleagues sought to explain its formation. “This is a molecule that doesn’t like molecules,” Schilke says, just as argon is an atom that doesn’t like atoms. This peculiar characteristic is turning out to be useful.
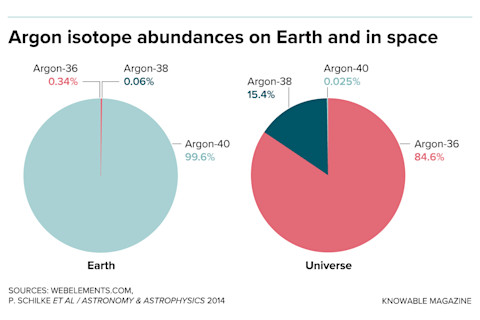
On Earth as it is not in the heavens: The argon in Earth’s air is nearly all made up of the isotope argon-40, but in space (as measured from the solar wind) a different isotope, argon-36, dominates. A mix-up between the two isotopes delayed the discovery of interstellar argonium.
Argonium’s Cosmic Origins
Based on standard calculations of how chemical reactions proceed in space, scientists know the formation of the interstellar argonium molecule requires two steps. First, a cosmic ray — a high-speed charged particle — strips an electron from an interstellar argon atom, making Ar+. Then that argon ion can steal a hydrogen atom from a hydrogen molecule (H2) to create argonium, ArH+, because the hydrogen atom is more attracted to the argon ion than to its hydrogen mate.
But argonium is fragile, and the same hydrogen molecules it requires for its formation can also destroy it. The noble gas molecule can therefore exist only where there’s just enough molecular hydrogen to create argonium but not so much as to tear it apart. This stringent requirement turns out to be handy for identifying which interstellar clouds aren’t likely to spawn new stars and planets.
Interstellar gas in our part of the Milky Way comes in two main types: atomic and molecular. The first and more common type consists primarily of individual hydrogen and helium atoms. Because atomic gas is diffuse, it rarely makes new stars. Instead, most stars arise in denser gas where atoms crowd together to create molecules.
It can be difficult to tell apart the interstellar clouds that consist mostly of atomic gas from those that consist mostly of molecular gas, and that’s where argonium comes in. “It’s a tracer of almost purely atomic gas,” Schilke says. In fact, although argonium is a molecule, it exists only in gas that’s 99.9 to 99.99 percent atomic.
Because cosmic rays lead to the creation of argonium, its abundance in interstellar space has also helped nail down the number of cosmic rays darting through the galaxy. “There are more cosmic rays than we thought before,” Gerin says. That’s important not only for future Captain Kirks wishing to minimize their exposure to the destructive radiation as they travel between star systems, but also to scientists studying the chemistry of the interstellar medium, because cosmic rays are the first step in the creation of other molecules as well.
The Universe’s First Molecule
Even after the discovery of interstellar argonium, astronomers continued their quest for the simplest noble gas molecule, helium hydride, the one that theorists had predicted decades ago. “This is the first chemical bond that formed in the universe,” says astrophysicist Stephen Lepp of the University of Nevada, Las Vegas.
The molecule arose because hydrogen and helium were the two chief elements to emerge from the big bang. At the beginning, the universe was so hot that any electrons either element managed to capture would immediately be stripped away by high-energy radiation generated by the extreme heat. But as space expanded, it cooled, and about 100,000 years after the big bang, each helium nucleus grabbed two electrons and became neutral. Put H+ and He together and you have the universe’s first molecule, HeH+.
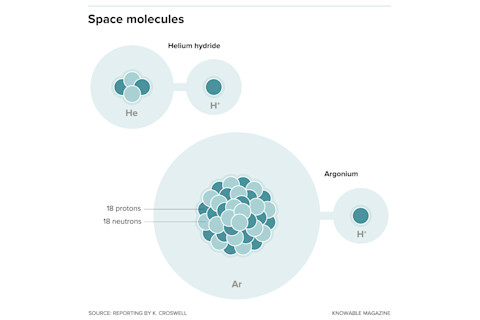
Helium hydride and argonium are the two noble gas molecules astronomers have found in space.
To this day no one has ever detected any helium hydride in the early universe; that would require the unprecedented feat of looking across more than 13 billion light-years of space to the dawn of time and discerning the faint spectral line that the molecule produces. In April 2019, however, astronomers led by Rolf Güsten of the Max Planck Institute for Radio Astronomy in Germany reported finding the long-sought molecule right here in the Milky Way.
Güsten’s team made the discovery not with a spacecraft but with a specialized airplane that flies above nearly all of the atmosphere’s water vapor, which blocks infrared radiation. The Stratospheric Observatory for Infrared Astronomy hunted for the coveted molecule using a telescope with a sensitive new high-resolution spectrometer. This instrument successfully detected the far-infrared signature of HeH+ at a wavelength of 149 micrometers.
Güsten and his colleagues succeeded by searching the same nebula where their predecessors had failed: NGC 7027 in the constellation Cygnus. Here, about 600 years ago, an aging star known as a red giant shed its atmosphere — something our own sun will do in about 7.8 billion years. This exposed the dying star’s hot core, which shines at a blistering 190,000 kelvins (340,000 degrees Fahrenheit) and emits extreme ultraviolet light that tears electrons from helium atoms, creating He+. Combine that with neutral hydrogen atoms from other parts of the nebula and you have HeH+. In the early universe it was the other way around — charged hydrogen and neutral helium — but the end result was the same: HeH+, the first molecule to form after the big bang.

For decades, astronomers have sought the lightest noble gas molecule, helium hydride, in the planetary nebula NGC 7027, shown in this composite image. In 2019, they finally reported success, detecting the molecule. Helium hydride is thought to be the first type of molecule to form after the universe’s birth. (Credit: William B. Latter (SIRTF Science Center/Caltech) and NASA/ESA)
William B. Latter (SIRTF Science Center/Caltech
“It’s the end of a long saga,” says Paul Goldsmith, an astronomer at NASA’s Jet Propulsion Laboratory who was not involved with the discovery. The detection proves that the calculations predicting the exotic molecule’s existence were correct, lending credence to expectations that the molecule did indeed take shape soon after the universe’s birth.
There might be other noble gas molecules, too. In space, neon atoms greatly outnumber argon, so neonium, or NeH+, could exist. If so, its abundance and the places it exists will further illuminate conditions in the interstellar medium. On the other hand, krypton is so rare that kryptonium probably poses little threat to any interstellar Superman, and xenon is rarer still.
But it’s a vast universe with temperatures and densities that vary wildly from place to place and differ dramatically from those on Earth. Somewhere, in the nook of some distant interstellar cloud, the most unlikely atoms may have come together to create molecules even more bizarre than any yet found, awaiting only an intrepid observer to detect their spectral signature in the depths of space.
10.1146/knowable-121119-1
Ken Croswell is an astronomer and author. In the 1980s he and Alex Dalgarno predicted that the interstellar medium should contain the molecule OD, which astronomers discovered three decades later.
This article originally appeared in Knowable Magazine, an independent journalistic endeavor from Annual Reviews.














