Larry Black kneels in the sandy soil beside a bushy orange tree flush with ripening fruit, his brow glistening in the hot Florida sun. He pinches a sprig of young leaves and pulls it in at eye level. “You see him?” Black says. “He’s tiny.” A grayish speck flutters off. It’s the Asian citrus psyllid — smaller than a grain of rice, but big enough to possibly destroy Florida’s citrus industry.
The tree’s yellow-blotched leaves betray a symptom citrus growers have come to expect. It’s sick. And so is nearly every mature citrus tree in the state.
Black’s family has raised oranges here since the 1850s. For five generations, they’ve faced hurricanes, frost and pests. But over the past decade or so, they’ve seen this tiny bug become their worst calamity, decimating the state’s iconic orange trees by ferrying a disease called citrus greening, or Huanglongbing (HLB) — the yellow dragon disease.
“Pre-HLB, a grower planted a grove of trees and expected them to live for a generation,” says Black, who runs Peace River Packing Co. in Fort Meade. “And that’s just not a reality anymore.”
Standing beside Black is Fred Gmitter, a citrus breeder and geneticist at the University of Florida. His skin is sun-weathered and freckled, forged by decades of walking groves just like this one.
Gmitter picks two leaves and holds them out. He explains that the bacteria behind citrus greening, Candidatus Liberibacter asiaticus, invade and clog a plant’s phloem — the internal plumbing system for circulating sugar. Sugar gets stuck in the leaves, messing with photosynthesis. The roots starve. Surviving trees often bear sour and misshapen fruit.
“It’s like an atom bomb going off in the tree,” Gmitter says.
At the industry’s height in 1997, Florida’s nearly 1 million acres of citrus could’ve covered Rhode Island. Growers harvested a whopping 244 million boxes. This year’s predicted haul: 46 million boxes, the worst since World War II, thanks to a one-two punch from greening and Hurricane Irma. The U.S. Department of Agriculture has spent nearly half a billion dollars fighting the disease, and yet these days, that jug of OJ in your fridge likely is mixed with Brazilian oranges.

Larry Black, a Florida orange grower, has planted a grove of Sugar Belles, a citrus variety more tolerant of a disease that's killing citrus trees around the world. (Credit: Ernie Mastroianni/Discover)
Ernie Mastroianni/Discover
But Florida growers see reasons for hope. Scattered among the sick and dead trees, Gmitter has found some strong survivors. These trees still get infected but show fewer symptoms and grow healthy fruit. Inside their genes, scientists are hunting for a cure.
“For the industry, immunity is the thing you’re looking for. That’s the long game,” says Tim Eyrich, the head researcher at Southern Gardens Citrus, one of the state’s largest growers. “And immunity is probably going to come through some type of genetics.”
Walk the juice section at your local grocer, and you’ll find bottle after bottle stamped “non-GMO.” This means the DNA of the ingredients inside haven’t been edited by science to include foreign genetic material. But despite the labels, many growers believe they won’t survive in the long term without a solution that includes genetically modified organisms (GMOs).
“Within 10 years, there might not be any orange juice left,” says Brian Staskawicz, a plant disease expert at the University of California, Berkeley. “So you ask the people, do you want a GMO orange tree, or you want no orange juice? Take your pick.”

Citrus greening is transmitted by the tiny Asian citrus psyllid. The disease was first detected in Florida in 2005 by state agriculture workers. (Credit: Michael Rogers/University of Florida/IFAS/Citrus Research and Education Center (left); Ernie Mastroianni/Discover)
Michael Rogers/University of Florida/IFAS/Citrus Research and Education Center (left
The choice might not be quite so black and white. A new gene editing technology called CRISPR lets scientists create genetic mutations in a more natural way that’s also faster and cheaper than previous techniques.
“It’s different from a classical GMO in that we’re not adding a genome from another organism,” Gmitter says. Instead, by knocking out a few existing genes, researchers are trying to engineer a tree resistant to greening.
This technicality — that it’s not transgenic, like putting a fish gene in a tomato — is leading U.S. government regulators to take a hands-off approach. No final decision has been announced, but so far regulators say the CRISPR’d crops are non-GMO. The agriculture industry could have the benefits of genetic modification without the stigma.
It’s not just oranges being infected. Pests and diseases destroy up to 30 percent of global crops. From bananas and tomatoes to wheat, rice and potatoes, a surprising number of common foods are in peril. And such blights disproportionately affect the developing world because fertilizers, pesticides and genetic engineering are either unavailable or prohibitively expensive.
Modern food is grown in monocultures — where crops are genetically very similar — to make harvesting easier. But that means when a disease strikes, all the plants get sick. In addition, globalization has meant that diseases can spread faster and farther, and the warmer temperatures from climate change can attract pests to new regions. Meanwhile, farmers must grow 70 percent more calories by 2050 to feed some 10 billion people.
CRISPR could help solve these problems.
Democratizing GMOs
Gmitter still remembers the day in 2005 when state agriculture workers found citrus greening in a backyard near Miami International Airport. Even before greening arrived, Florida growers were worried about it. A century earlier, the disease had devastated groves in China.
As it spread across Florida — as well as Brazil, China and dozens of other countries — Gmitter and a small team of international citrus scientists persuaded industry groups to give them about $6 million to sequence the orange tree’s genome. “If we have the blueprint for the citrus tree — if we have the catalog of genes — this becomes a toolbox,” Gmitter recalls thinking. Inside that toolbox, he hoped to find a solution.
By the time they released the first citrus genome sequence in 2011, the cost of sequencing technology was already plummeting. The next year brought the birth of a radically new way to genetically engineer life: CRISPR.
Each time a virus attacks bacteria, those bacteria save a snippet of the invader’s DNA in their genome. They use this snippet as a kind of mugshot to spot and remove the virus when it attempts another invasion. Molecular biologist Jennifer Doudna of UC Berkeley, working with French biologist Emmanuelle Charpentier, discovered that this natural defense system could also be employed as a kind of DNA scissors. This tool, called CRISPR-Cas9, can target and cut with incredible efficiency.

Nearly all of Florida’s orange trees show signs of citrus greening. (Credit: Ernie Mastroianni/Discover)
Ernie Mastroianni/Discover
So far, the hype has centered on combating human disease, like when an American-led team of scientists corrected heart disease-causing genes in a human embryo last year. But CRISPR has already pushed well beyond biomedical breakthroughs. Researchers at Penn State University, for example, edited the genes of a common white button mushroom so that the fungi resisted browning. On the livestock side, biologists at the University of Missouri used CRISPR to breed a litter of pigs that are unharmed by a disease that costs the industry $600 million each year.
Hundreds of millions of dollars are now going toward applications in agriculture. From DuPont Pioneer to Monsanto, major seed corporations are trying to cash in. But plant disease experts say the real revolution will come when gene editing is used on crops overlooked by large agriculture companies.
Doudna sees CRISPR as the democratization of gene modification, where we could even have gene-edited plants growing in our backyard gardens. “It is such an accessible technology,” she says. Last year, the Innovative Genomics Institute (IGI) at UC Berkeley — a lab co-founded by Doudna — launched a $125 million initiative to unleash CRISPR on agriculture and other areas outside of medicine. “There may be even more applications for CRISPR in agriculture than there are in human biology,” says Staskawicz, whom Doudna tapped to lead IGI’s crop efforts.
A Plant From One Cell
Breeders spent more than a century creating genetic crosses to increase disease resistance, Staskawicz says, yet scientists only recently figured out how it worked: Plants and animals both rely on a major class of disease resistance genes.
Many bacterial diseases infect plants using what scientists call a type-III secretion system. That’s a rather boring name for a robust, destructive little molecular machine. This machine’s main objective is to inject proteins that disarm the plant’s immune system. But the battle isn’t totally one-sided. Once the disease resistance genes kick in, they trigger a cascade of effects to fight off the infection.
You can get those disease resistance traits through crossbreeding, but doing so also pulls in genes that could diminish the crop. CRISPR’s precision lets scientists select specific genes from a plant’s relative — wild or domestic — and insert only the desired traits. Scientists can also simply knock out a gene that leaves a plant susceptible to disease.

UC Berkeley researcher Myeong-Je Cho is learning to use CRISPR on a variety of plants. Credit: Eric Betz/Discover)
Eric Betz/Discover
One of the biggest challenges has been delivering the CRISPR components into seeds. And in a newly minted lab at the IGI, plant scientist Myeong-Je Cho is trying to figure out how to use CRISPR on plant seeds, including those from the cacao tree, which is hobbled by a disease that threatens livelihoods in the developing world. Before joining IGI in 2016, Cho worked at the ag corporation DuPont Pioneer. IGI hired him because of his approach to using CRISPR on seeds.
Past techniques inserted CRISPR into cells using Agrobacterium, a bacteria that can also carry the location for the scissors to cut. But that method is still transgenic. Cho’s approach puts CRISPR directly into cells. Staskawicz says it’s a significant advance.
To demonstrate, Cho grabs a scalpel and dissects a tiny flower. Donning a white lab coat, he cozies up to his weapon of choice: a gene gun. There’s no pistol grip or trigger; it’s just a tiny box that holds a petri dish full of plant embryos. Instead of bullets, the gun shoots hundreds of thousands of gold particles coated in CRISPR components. He fires and — pop! — they splatter like a shotgun blast. The particles penetrate the plant cells inches below, delivering CRISPR.
“If you look at it under a microscope, there are many, many holes,” Cho says.
The technique relies on a remarkable capability of plant cells called totipotency. In humans, only stem cells have the ability to become any body part. But for plants, each and every cell can form everything.
“A single cell has the potential to become a whole plant,” Cho says.
If Cho can make CRISPR work on cacao and other plants, the new crops will keep the same properties as their parents — the refined product of thousands of years of breeding — but exclude the genes that make the crops susceptible to disease. After he’s done with the gene gun, Cho shows off sparkling white refrigerator-sized incubators full of petri dishes. Inside each perfectly stacked container is a clump of what looks like pre-chewed food. Many sport little green shoots that’ll grow up to be genetically modified broccoli, rice, wheat, cacao, pepper and tomato. Each is part of IGI’s efforts to fix one crop problem or another.
“The technology is robust, and it’s simple,” Staskawicz says. “A lot of people can do it, and you don’t need fancy equipment.”
Frankenfood Freakout
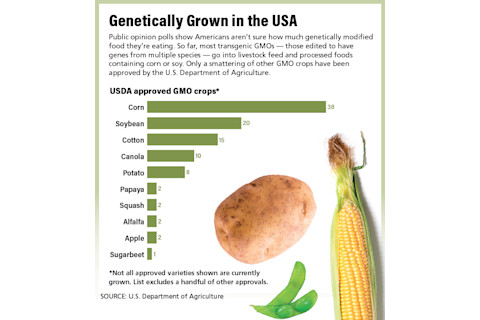
Most of us don’t think about it, but we eat GMO foods every day. Almost all American-grown corn and soybeans come from genetically modified seed. The two crops are used as sweeteners and fillers in an amazing array of processed foods.
Wheel your cart around a supermarket, and you’ll push past aisles of GMO foods, such as breads, cereals and crackers, as well as yogurt, milk and meat. Even cheese is made from genetically engineered rennet — the enzyme that curdles milk — instead of traditional rennet from animal stomachs.
But not long after engineered corn and soybeans hit the market in the mid-’90s, the term GMO got tangled together with concerns about pesticides and patented seeds. And there’s good reason for that. The first wave of genetically engineered foods was all about farmers’ needs (like crops that withstand pesticides and net higher yields) and corporate profits (from selling those pesticides).
Citrus Greening

(Credit: Ernie Mastroianni/Discover)
Ernie Mastroianni/Discover
Other names: Huanglongbing (HLB), yellow dragon disease.
Caused by: Candidatus Liberibacter asiaticus bacteria, spread by the Asian citrus psyllid insect.
Affected: Citrus plants, including oranges, grapefruits, lemons, limes and tangerines.
Symptoms: Yellow shoots; dark aborted seeds; mottled or patchy discoloration to leaves; mature fruit that is small, hard, misshapen, partially green and falls from its stem prematurely; bitter taste.
Cure: None. Infected trees usually die within a few years.
Ernie Mastroianni/DiscoverThe public disdain created a bizarre supermarket reality — a GMO-free zone in the produce section. The agriculture industry is convinced we’ll accept genetic engineering in processed foods yet recoil at GMO whole foods.
There is one exception: the papaya. Some 30 years ago, Hawaii’s papaya industry — like the citrus industry today — was decimated by an unstoppable disease. Cornell University scientist Dennis Gonsalves came up with a GMO papaya that survived the virus, and he gave away the seeds for free. The plant saved the industry.
Interestingly, surveys show Americans aren’t sure what they think about GMOs. A 2016 Pew poll revealed that the vast majority has heard just “a little” or “nothing” about the subject. About half of Americans believe they eat some or no genetically modified food. But among the 16 percent who say they care deeply about GMOs, the perception is largely negative. That’s despite the scientific consensus — including a large-scale report from the National Academy of Sciences in 2016 — that GMOs are safe and nutritionally identical to conventional crops.
Marketing hasn’t helped the stigma. “Non-GMO” labels adorn all manner of products, regardless of whether a transgenic version exists. Even the orange juice industry — whose farmers are helping fund a GMO solution — labels containers.
“[Food producers] see it as a revenue driver,” says Tim Eyrich, vice president of research at Southern Gardens Citrus. “That’s why we see non-GMO Himalayan salt, whereas the last time I looked, sodium chloride doesn’t have DNA. That’s a marketing thing. And that’s an education thing. But people buy it.”
That confounds plant scientists, who see gene editing’s potential to fix all manner of ills, from crop diseases to pesticide overuse.
“Here’s the real problem: We need food,” says Staskawicz, pointing to the world’s growing population. “And you’ve got to do it in some sort of environmentally sustainable fashion. You’ve got to really reduce farmer inputs — things like pesticides and fertilizers. These things all contribute to global warming.”
A Cautionary Tale
Molecular biologist Diana Horvath understands how hard it is to bring GMO produce to market. She gave up her venture capital job to cofound the non-profit 2Blades. Her goal was to move plant disease breakthroughs from the lab to the field.

Nian Wang, a microbiologist at the University of Florida’s Citrus Research and Education Center (CREC), examines containers of edited citrus stored in the incubating room. He and his team have identified 13 genes that may be linked to citrus greening. (Credit: Ernie Mastroianni/Discover)
Ernie Mastroianni/Discover
In 2004, she found a poster child for a “good” GMO. Tomato farmers had been battling a disease called bacterial leaf spot, which shrivels plants. Growers try to control it with copper-laden sprays, even though the bacteria is now resistant.
But peppers, a close relative to tomatoes, contain a gene that gives them immunity to the disease. Staskawicz’s lab found a way to insert that gene into tomato plants, making them immune. During field trials, Florida farmers grew more food without using the traditional chemicals. And yet GMO tomatoes didn’t pan out. Receiving U.S. Department of Agriculture approval is expensive, and growers wouldn’t gamble on a crop the public might reject.
But USDA approval isn’t needed for CRISPR’d foods. And regulators said Penn State’s non-browning mushrooms aren’t GMOs because there’s no “introduced genetic material.”
Of course, consumer sentiment could halt CRISPR’d crops anyway. And growers quickly backed off 2Blades’ tomatoes even though U.S. law doesn’t require obvious GMO labels.
Citrus could provide the test case. Some Florida growers have sold their citrus fields to developers, while others have simply abandoned their orchards. But those who are still in the game — such as Black, the Florida grower — know all about the latest tech, including CRISPR. “Most growers look forward to a genetic solution,” Black says.
CRISPR Hope
Just outside microbiologist Nian Wang’s cramped office at the University of Florida’s century-old Citrus Research and Education Center in Lake Alfred, a cadre of young scientists work diligently along lab benches. Parts of the building date back to the 1930s. Yet in these crowded and somewhat dated quarters, Wang and his team have pushed the limits of scientific knowledge.

Citrus shoots grow in a petri dish at CREC’s Core Transformation Lab, where scientists use gene editing in the fight against citrus greening. (Credit: Ernie Mastroianni/Discover)
Ernie Mastroianni/Discover
Plant Genetics Through Time
Humans have altered their food for thousands of years.
>10,000 B.C. | First Farmers Humans begin domesticating plants for food.
Early 1900s | Plant breeding Becomes Science Sir Rowland Biffen crosses breeds to create disease-resistant wheat. Scientists can now select for traits instead of relying just on chance.
1994 | First Commercial GMO Calgene (today owned by Monsanto) launches slow-ripening Flavr Savr tomatoes. It’s a hit with consumers, but soon is canceled in part due to high costs.
1999 | Opposition Grows A Cornell University study implies GMO corn pollen endangers monarch butterflies. Experiments by the USDA rebut the finding, but the perception sticks.
2003 | Fresh Regs The European Union passes strict rules on GMOs. Many EU countries later ban farming them.
2012 | New Edits Scientists show they can edit genes with CRISPR, and it’s used on plants the following year. The method outshines existing tech.
2016 | No Difference A two-year study by the National Academy of Sciences finds no significant difference between GMOs and non-GMOs in risk to health or environment.
All citrus plants are genetically very similar, but that doesn’t mean the genome is simple. Wang’s team has found it challenging to employ CRISPR’s DNA scissors. “Citrus is not the model system,” says Vladimir Orbovic, who helps Wang and other citrus scientists conduct their experiments in the center’s Core Transformation Lab. “It’s a very complicated crop.”

University of Florida citrus breeder and geneticist Fred Gmitter (left) has created several new citrus varieties now stored in an enclosed facility that isolates them from insects and other outside contaminants. (Credit: Ernie Mastroianni/Discover)
Ernie Mastroianni/Discover
Ironically, scientists haven’t been able to grow the bacteria effecting greening in a lab, making it harder to study. Another hurdle is that greening is a relatively new disease. Gmitter and his colleagues have studied the evolutionary history of citrus. Their results show that, while the plant was first domesticated in Asia thousands of years ago, greening showed up only in recent centuries. The disease is so new that even wild trees aren’t immune.
But Orbovic says that if they can get CRISPR to work in a complicated system like citrus, their methods could prove extremely useful for editing other crops, too. And over the past year, they’ve had a breakthrough. Wang’s team has identified 13 potential genes that cause citrus to be susceptible to greening. His team is now trying to knock out those genes with CRISPR.
“We don’t really know which one is the right one,” Wang says. “So we do all of them, and hopefully we get one of them right.”
As each plant is edited, the fruits of their labor are stashed next door in a makeshift incubating room. The room is a mess. Over-the-counter grow lights nurture a mélange of petri dishes and vials stacked on cardboard atop discount-store shelving units. A citrus sapling inside one of these vials — sealed with plastic film and a rubber band — could be the salvation of an industry. But it will take awhile to find out.
Citrus trees take years to reach maturity. After editing an orange tree’s cells, Wang’s team will have to wait as long as two years to expose the plant to citrus greening. Only then will researchers know the tree is immune. Even then, they will have to wait another couple of years for the immune plant to produce fruit to ensure the oranges still taste good.
(Credit: Soybean: NattapolStudiO/Shutterstock; Potato: Danza/Shutterstock; Corn: Alex Staroseltsev/Shutterstock)
Soybean: NattapolStudiO/Shutterstock; Potato: Danza/Shutterstock; Corn: Alex Staroseltsev/Shutterstock
But Wang’s work has given the industry some hope. A short walk from his office building is a greenhouse repository called “the ark.” This is where the saplings go after outgrowing their vials. Inside, Wang shows off a healthy young citrus tree. His team used CRISPR to make it resistant to citrus canker, a disease that’s simpler to tackle than greening.
Farming Realities
About an hour south of the Lake Alfred labs, Black parks his pickup in front of a grove of freshly planted trees. They’re Sugar Belles and Bingos, new varieties bred to compete with California Cuties.
He can’t afford to wait for a CRISPR solution; he’s got to plant today, and these varieties are more tolerant of greening. He can still turn a profit farming citrus if he can keep the trees alive for 15 years.
He recently almost lost them. After Hurricane Irma, Black returned to discover 90 mph winds had blown over 4,000 young trees. His company had to restake each one. But Black shrugs it off, recalling generations of calamities that have reshaped the industry.
“This is just agriculture,” Black says. “It happens. Today’s problem always seems worse than all those that have come before.”















