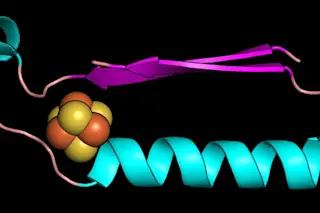
While the proteins that create life on Earth seem to vary indefinitely, there are a few parts they all have in common. Researchers say they have discovered which simple proteins were present when life on Earth first began to diversify, as described this week in a study published in PNAS.
The authors say these prehistoric proteins are smaller pieces of molecules that we know today as “enzymes” — materials that facilitate chemical reactions in living organisms. When isolated on their own, it appears the fragments act as enzymes, too. Two pieces in particular — one that binds iron to sulfur and one that helps nucleotides, the core pieces of DNA, connect — might be two of the oldest enzymes on Earth.
Still present in enzymes working today, these two fragments are the core pieces in the building blocks of life. They can function alone, but researchers say they allowed for ...