Growth rate is a fundamental aspect of life, a metric that can separate biology’s winners and losers. Even a small advantage can lead to complete dominance in short order, given the exponential scaling patterns of biological growth. Calculating growth rate is pretty straightforward when you’re looking at plants or animals, where it’s possible to measure an organism’s mass with relative ease. But what about microbes? How can minuscule changes in a cell’s mass be measured when the whole organism weighs just a picogram (10-12 grams)?
New Microfluidic Device Allows Researchers to Measure Cell Growth...by the Picogram
Oct 12, 2016 5:24 PMOct 15, 2019 4:19 PM
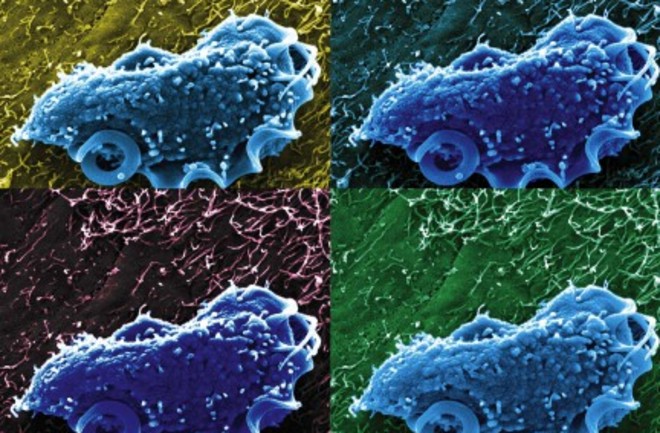
Understanding how growth rates of individual cells varies is a key priority for microbiologists. (Image: NIH/Aleksey Chudnovskiy and Miriam Merad, Icahn School of Medicine)
Newsletter
Sign up for our email newsletter for the latest science news
0 free articles left
Want More? Get unlimited access for as low as $1.99/month
Stay Curious
Sign up for our weekly newsletter and unlock one more article for free.
View our Privacy Policy
Want more?
Keep reading for as low as $1.99!
Already a subscriber?
Find my Subscription
More From Discover
Stay Curious
Subscribe
To The Magazine
Save up to 40% off the cover price when you subscribe to Discover magazine.
Copyright © 2025 LabX Media Group
