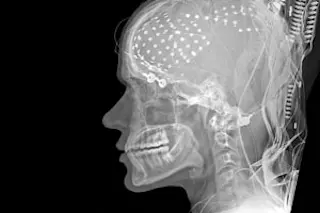
Deep brain stimulation can now be used to treat obsessive compulsive disorder, or OCD, which causes uncontrollable worries and anxiety in its sufferers. Medtronic's Reclaim deep-brain stimulation (DBS) device received approval from the Food and Drug Administration after a study of 26 patients with severe OCD that showed a 40 percent reduction in symptoms after a year of deep brain stimulation therapy. All the patients had tried and failed other therapies [Chicago Tribune]. The Reclaim device is implanted under the skin of the chest and then connected to four electrodes in the brain. The electrodes deliver steady pulses of electricity that block abnormal brain signals [AP]; the device is controlled by a battery-run component outside the body. Hooman Azmi, a neurosurgeon at Hackensack University Medical Center, said, "This is essentially like a pacemaker for the brain" [WebMD Health News].While the device has previously been used to treat Parkinson’s Disease and ...
Obsessive Compulsive Sufferers May Find Relief With a "Brain Pacemaker"
Explore how deep brain stimulation therapy offers hope as an OCD treatment, significantly reducing symptoms with the FDA-approved device.
More on Discover
Stay Curious
SubscribeTo The Magazine
Save up to 40% off the cover price when you subscribe to Discover magazine.
Subscribe