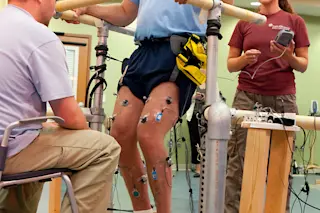
Rob Summers is standing up. Two feet on the ground, legs straight, hips squared. He has done it thousands of times before — out of bed in the morning to practice with his championship-winning collegiate baseball team, or up from the couch to get a snack.
Most memorably, he stood up on a July night in 2006 to walk out the door and over to his parked car on a street in Portland, Ore. Standing next to his Ford Explorer, he saw the lights of another vehicle approaching from behind. It was coming fast — too fast.
Before he could get out of the way, the car threw him to the ground, and the driver fled what was a gruesome scene: Summers lay on the asphalt in a pool of blood, the victim of a hit-and-run that severed the connection between his brain and spinal cord and paralyzed him from ...