Trudging to his car through the midwinter gloom, David Barker is heading home. He drives fast past winter-drab fields and deep into the English countryside. Along the way, he opines on, among other things, the role of pubs in British life, the Norman conquest of England, and the exploits of one of the area residents, Sting. If this courtly physician has anything in common with Sting, it is not celebrity. A decade ago, the "Barker hypothesis" was almost unknown, drowned out by the thunder of excitement over the genetics revolution. Heart disease, cancer, and obesity were considered mostly a matter of DNA and adult lifestyle. Barker, who heads the Medical Research Council Environmental Epidemiology Unit at the University of Southampton, broke rank by arguing that health and disease have more complex roots. Conditions in the womb and in early infancy, he said, "program" the way our kidneys, liver, pancreas, heart, and brain develop, and how they function later in life. When a fetus must adapt to a poor environment in the womb, or when infants are exposed to malnutrition or infection shortly after birth, permanent and even lethal damage is done. This view challenges not only genetic dogma but also the very foundation of public-health doctrine. "One of the most threatening things about fetal programming," Barker says, "is that it means that God may reward you less than you think for changing your lifestyle." Once derided as something Aldous Huxley might have dreamed up as a sequel to Brave New World, Barker's theory is now widely embraced, even by many of his early critics. The National Institutes of Health has pledged to plow $3.8 million into studying the fetal origins of adult disease, and partially as a consequence of this largesse, interest in the field has burgeoned. In the past couple of years, the number of scientific papers published on the topic has gone from a trickle to a steady stream, and at a recent Society for Experimental Biology conference, sessions on fetal programming were crammed. "I have absolutely no doubt that fetal programming is extremely important," says Claude Lenfant, director of the NIH's National Heart, Lung, and Blood Institute in Bethesda, Maryland. "It could explain so many things that we do not now understand. We know that most diseases result from a mismatch between genes and environment. The question is, when does the 'environment' piece begin—when you take your first breath of air, or earlier? I say it's earlier. Like Barker, I say it's in the womb." To understand fully Barker's thinking, it is first necessary to let go of the cherished myth that mothers-to-be sacrifice all to the next generation. The bitter truth is that growing teenagers and undernourished or vitamin-depleted women are less able than other mothers to nourish their fetuses. When pickings are slim, the fetus loses out. A malnourished fetus must perform a sort of triage, diverting nutrient-rich blood to the most vital of vital organs—first the brain and then the heart—thereby shortchanging other parts. Because these organs grow at different rates—some continuing to develop into the first few years of childhood—the impact varies depending on when and what sort of malnourishment occurs. The baby may be born looking and acting healthy but with a liver, kidney, or pancreas subtly compromised in a way that will show up later in life. And a woman who begins pregnancy in a less-than-healthy state may have difficulty passing nutrients through the placenta, depriving her baby even if she eats well.

David Barker sits among the thousands of health records that helped him make a case for the impact of fetal nutrition on adult health. "When I talk about the importance of nutrition in fetal health," he says, "every mother knows it and looks at me pityingly when I explain it."
"The fetus and the young infant are very plastic, and the fact is we know very little about these periods of life," Barker says. "But what's painfully obvious is that what happens during these phases has very much to do with adult health." Educated at Oundle, one of England's most exclusive private schools, Barker acquired from an early age both a love of natural history and a restless streak. After earning a medical degree at Guy's Hospital in London and a Ph.D. in epidemiology at the University of Birmingham, he and his first wife, Mary, packed up their four young children and moved to Uganda. Barker knew next to nothing about Africa and even less about buruli ulcer, the horrifying condition he had set out to investigate. Buruli is spread by the bacterium Mycobacterium ulcerans, which spits a toxin into the body's tissues, causing swelling, baseball-size ulcers, and if left to do its work, loss of limbs, eyes, and vital organs. There is no drug treatment, and early surgery to cut out infected tissue is the only cure. "The popular belief was that the bacteria was carried by mosquitoes," Barker says, but he had doubts. He had mapped the course of buruli in Uganda and found that the disease correlated with proximity to the swamplands created by newly flooded regions of the Nile. Barker wandered down to the local bog to have a look and recognized patches of Echinochloa pyramidalis, a scratchy swamp grass. He thought the culprit microbe might be an aquatic organism that slipped though abrasions caused by the grass, but unfortunately he had no time to confirm the suspicion. "This was 1972," he says, when soon-to-be "president for life" Idi Amin Dada was turning Uganda into his own private killing grounds. "We put our pet dogs down with lethal injections," Barker says. "And we fled." Barker never did track down the precise carrier of buruli bacteria, which remains a mystery today. But he left Africa convinced that accepting conventional scientific wisdom was not always the best way to decode the etiology of disease, a conviction that, a dozen years later, led to the theory that would make his name. Barker was by then a professor and director of the Medical Research Council Environmental Epidemiology Unit, where he had cultivated an expertise in the geography of chronic disease. While looking over a newly edited disease map of Great Britain with his colleague, statistician Clive Osmond, he noticed a striking geographic trend in heart disease rates. With the notable exception of London, men ages 35 to 74 in poor industrial regions of Wales and northern England had substantially higher rates of heart disease than did men in wealthier southern regions. Barker thought this odd because the prevalence of heart disease tends to rise with increasing prosperity. Moreover, the men in the high-disease counties ate no more fat, used no more tobacco, and if anything got more exercise than did men elsewhere in Great Britain. Clearly, diet and lifestyle were not enough to explain the discrepancy. "The thing about chronic disease is that it's 30 to 50 years in the making," Barker says. "To get a clear picture of what was going on in these men, we had to take a look at them as babies." Barker decided to track down what, if anything, in the early lives of these men could predict their health as adults. His staff scoured archives and hospitals throughout Britain, looking for maternity and infant-welfare records. They found plenty—in lofts, boiler rooms, and flooded basements. The records stretched from 1945 back to the early years of the 20th century. Most were erratic and incomplete, but in Hertfordshire, a green and fertile county just north of London, the records were kept with meticulous care, thanks largely to the efforts of Ethel Margaret Burnside, a dedicated nurse and midwife and the county's first Chief Health Visitor and Lady Inspector of Midwives. Burnside organized a sort of midwife army that not only helped with deliveries but also recorded on follow-up visits the health of a baby and whether it was breast- or bottle-fed. The discovery of these records in 1986 made it possible to relate people's early growth and diet to their health in later life. Barker's team tracked down 5,654 males from the Hertfordshire babies, most of whom were by then in their mid-seventies. Comparing adult records provided by the British National Health Service with the infant data from Hertfordshire, Barker discovered that Hertfordshire babies born weighing less than 5.5 pounds were significantly more likely to develop coronary heart disease as adults than were infants of normal weight. The maps had hinted that something in the womb was marking some children for life, but this finding, says Barker, "clinched it." "The old model of adult degenerative disease was based on an interaction between genes and adult environment," says Barker. "The new model that is developing will include programming by the environment in fetal and infant life." In 1989 Barker and Osmond published a landmark paper in the British journal The Lancet, correlating low weight both at birth and at age 1 with an increased incidence of cardiovascular disease in men. Other scientists duplicated this finding, and Barker and others have since found correlations between insufficient fetal nutrition and other conditions such as high blood pressure, diabetes, obesity, and kidney failure. Barker and other adherents of the fetal-programming theory say that deprivation in early life is an important and often overlooked risk factor in human health. For example, the human kidney grows most rapidly between 24 and 34 weeks of gestation, and if the fetus is malnourished during this critical window, the kidney's structure and function may be permanently altered. By contrast, the human liver is plastic for four years after birth, presumably to help accommodate the infant's change in diet from mother's milk through to solid food. Animal studies have shown that during this period of important growth, the liver is vulnerable to effects that can compromise its long-term function, including clearing cholesterol from the blood. That could explain why early malnutrition has been linked to adult heart disease. "When it comes to fetal health, we are talking not only about getting enough calories but about balance," Barker says. "You need a balance of nutrients. In the third world, a shortage of micronutrients like vitamin A or certain minerals can cause problems. In the West, it might be too much of a bad thing. We think that sweet drinks suppress placental growth, so this is very definitely a problem. A mother drinking a gallon of Coke a day will certainly contribute to the nourishment of her baby—but it's not the sort of nourishment that results in good health." Obesity has in the past decade exploded around the globe, a pandemic not entirely accounted for by changes in lifestyle. Infants whose mothers were severely undernourished in the first two trimesters of pregnancy are more likely than other infants to be obese as adults. And those who were severely underweight as newborns show a tendency toward diabetes if they become obese as adults. Scientists believe that these children have been programmed in the womb for a life of scarcity. Such children may be better able to endure famine, but they are not prepared to handle a diet high in fat and calories. Sad evidence of this is offered by the devastatingly high rates of obesity, diabetes, and heart disease in parts of India, Mexico, the Pacific Islands, and other regions of the world undergoing the so-called nutrition transition. In these areas, a sudden uptick in lifestyle collides with a society weaned on poverty. By contrast, Barker points to the south of France, whose population enjoys both a luxurious diet and among the lowest heart disease rates in the developed world. Barker says this so-called French paradox has its roots in a tradition dating back to the fall of the Second Empire in 1871, when the French government resolved to increase the vigor of its army by improving the health of its mothers. Since then, excellent prenatal care and well-nourished mothers have, Barker says, made the French resistant to heart disease. "The French paradox," Barker says, "is no paradox." Barker finds support for his theory even in the United States, among the richest and best fed of nations. Rates of heart disease in this country are falling, which Barker attributes in part to improved nutrition in the womb. Obesity, however, is rising precipitously, not only in the United States but also in other wealthy Western nations. Recent studies show that infants born to obese—and, in particular, obese and type II diabetic mothers—are more likely than other infants to be born big and to become obese and diabetic as adults. Scientists suspect that this is due to the mother's inability to regulate blood sugar and insulin levels, which cross the placenta and overwhelm—and alter—the fetal pancreas, making it less able to recognize and respond to insulin. In animal studies, this change appears to be permanent: Fetal rats injected with insulin show a lessening of responsiveness to the hormone as adults. Unless modified by diet or some other means in mothers of successive generations, this vicious cycle of ill health may continue to spiral indefinitely.
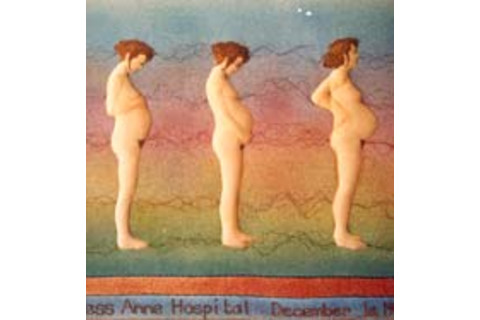
David Barker's wife, textile artist Jan Barker, created a large embroidery, now in a Southampton hospital, documenting the pregnancy of their daughter Rebecca. This section shows the last three of five stages.
While Barker's hypothesis has enjoyed growing support over the past decade, some continue to argue that it overstates the case. Michael Kramer, a pediatrician and perinatal epidemiologist at McGill University in Quebec, argued in a 1996 commentary in The Lancet that Barker tends to ignore data that contradict his thesis and that there is no shortage of published studies whose results do not support the fetal/infant-origins hypothesis. Kramer says that while he does believe "there is an association between impaired fetal growth and adult chronic disease," he is not convinced that this association means that fetal conditions determine or even affect adult health. Others note that Barker's theory does not explain why normal-weight women consuming a balanced diet may also have low-weight newborns. In these cases, they argue, the problem may stem from genes or environmental influences controlling the development of the placenta, which regulates the transfer of nutrients from mother to fetus. David Phillips, an endocrinologist and a colleague of Barker's, concedes that epidemiology can never prove causation but that "20 to 30 years or more of animal work examining the adverse effects of exposures in pregnancy" have made Barker's epidemiological findings all but irrefutable. Recently, a member of the Southampton group showed that rats born to mothers fed a low-protein diet have high blood pressure. Other scientists have shown that animals deprived of protein or certain vitamins in the womb grow smaller livers and kidneys and less-flexible blood vessels. "We've found evidence in both sheep and rats that if you produce very mild changes in maternal diet, you can mess up the vascular and endothelial cells, which dictate constriction of the blood vessels, and also mess up the hypothalamic pituitary axis, which is involved in almost everything," says Mark Hanson, a physiologist and director of the Southampton Centre for the Fetal Origins of Adult Disease. Whether this animal data can be extrapolated to humans is uncertain, but Barker is not relying solely on animal studies to vindicate his ideas. Since 1998 Barker's team has conducted a survey of 12,000 Southampton women between the ages of 20 and 34. They are monitoring the women's diets, body composition, and vital statistics, and waiting patiently for what they hope will be a healthy number of them to get pregnant. So far 1,500 have obliged, among them Lynne Allan, a 29-year-old cartographer who drops in for her checkup the day I visit. Lynne is eight months pregnant and remarkably good humored as she is scanned, questioned, and measured for nearly two hours. "What we're doing here is using a sledgehammer to crack a nut," says study coordinator Hazel Inskip, a statistician. "Epidemiology is the most powerful tool we've got, but the birthrate here is so low that we're forced to follow this large group to get at the data we want. Since you can't experiment on people, you have to observe them, and that takes time and an extraordinary level of commitment on everyone's part." By monitoring women's health before, during, and after pregnancy, and following their children, the Southampton group hopes to tease out what factors in the mother's nutrition affect fetal development and child health. "What we really want to find," says Southampton public-health specialist Catherine Law, "is a way to effect change. And the changes we are talking about require long-term reconsideration of behavior and social influences and focusing on the promotion of health rather than treatment of disease. Observational science such as this is by its nature uncertain, and for that reason there has been a perception that it's not 'real science.' But there is no question that the smaller you are as a baby, the riskier it is for you to grow large as an adult. That risk is very real." Barker has little patience for those who refuse to accept what he considers to be the patently obvious fact that health has its genesis in the womb. "American epidemiology has gone off on a boil, gone off to rooms filled with paper and no patients," he says. "Everyone has an Uncle Charlie who lived a grand life, drank, ate up a storm, and died at 100, while another guy lived a blameless life and died of coronary heart disease at 45. We can't blame everything on genes and go to sleep, because genes don't quite explain it either. People have to open their minds to the terrible possibility that we might be right—that the important events in the development of a child happen not at conception but years before. We don't need any more whacking big insights on this—we know that it's true. All we need now is for others to join the show."

The refrigerators of four participants in the Southampton Women's Survey—the world's largest study tracking links between the health of mother and fetus. Since 1998 the survey has recorded the diet and lifestyle of more than 12,000 young women in Southampton, England. Participants are given extra checkups and scans when they get pregnant. Photographs by Magda Segal.