Wearing white jeans and a navy shirt bejeweled with glittering stars, 15-year-old Paizley Carwell-Bowen lounges in the living room of her family’s North Hollywood apartment. She seems like a typical bubbly teenager —she chats and giggles with a girlfriend, she dreams of being a pop star—but she has a troubled past. “Sometimes I’d see the devil,” she says.
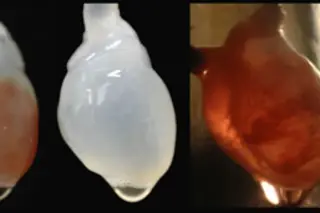
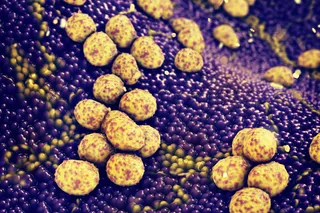
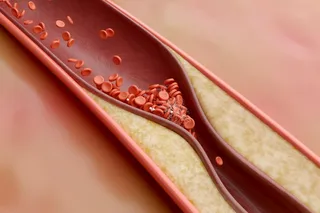
Paizley’s disturbing visions started after she had a stroke at age 6. The stroke was just one complication of sickle-cell anemia, the hereditary disease that has haunted her since infancy. Most common among people of African descent, the disorder causes oxygen-carrying red blood cells, which are normally flexible and round, to become rigid and take on a crescent (or sickle) shape. Sickle cells have trouble squeezing through fine blood vessels to deliver oxygen to the body’s tissues and organs. Instead they clump and choke off blood flow, causing intense pain as bits ...