This article appeared in the June 2021 issue of Discover magazine as "Looking Into Lithium Ion Batteries." Subscribe for more stories like these.
Lithium-ion batteries: They’re in almost every phone and laptop, they’re powering zero-emission transportation, and they’re making it easier to transition to wind and solar energy. Let’s demystify how these ubiquitous black boxes store energy — and why they can’t last forever.
Every battery has two core parts, an oxide electrode and a graphite electrode. The two electrodes are arranged in symmetrical, layered crystals. You can think of each as a Jenga tower whose stability comes from crisscrossing blocks. Rather than wood blocks, every other layer in the graphite electrode tower would be graphite; in the oxide electrode tower, it would be metal oxide. The remaining blocks would be ions of a soft metal called lithium, one of earth’s most widespread elements.
In a lithium-ion battery, these ions move between the two electrodes. When you charge your battery, electricity causes the lithium ions and an electron from the oxide electrode to move to the layers of the graphite electrode. When you use your phone or electric car, those electrons power an electric charge as they, along with the lithium ions, now naturally move back to the layers of the oxide electrode.
Imagine you’ve plugged your battery into an outlet to charge. The negative charges of electricity attract positively charged lithium ions to the graphite electrode. If you picture your battery as Jenga towers, you’d start with several empty spaces in the graphite electrode tower. Then, as the graphite electrode attracts the lithium ions, you slide out the lithium blocks from the oxide electrode tower and into the graphite electrode tower. Once all the spaces are used up on the graphite electrode, your battery is fully charged.
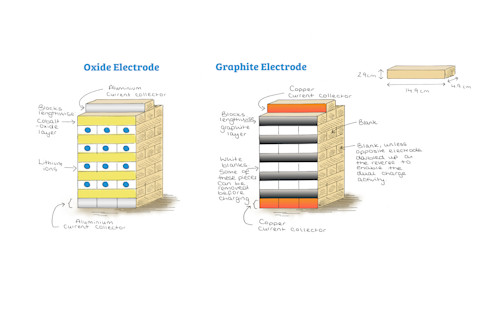
(Credit: Lizzie Driscoll/University of Birmingham)
Lizzie Driscoll/University of Birmingham
Each time you slide blocks into a Jenga tower, other blocks shift. Similarly, when lithium travels between the oxide and graphite electrodes, it distorts the neat layers around it. Eventually, the spaces where lithium once fit are no longer stable, and your battery begins to lose its ability to store energy. Elizabeth Driscoll, a chemistry Ph.D. student at the University of Birmingham, uses Jenga to teach battery science. Overeager students who move blocks too quickly, she says, exemplify what happens when you charge a lithium-ion battery too fast: The electrodes collapse and can no longer hold lithium. If lithium can’t move, the battery can’t store energy and it’s time to buy a new one.