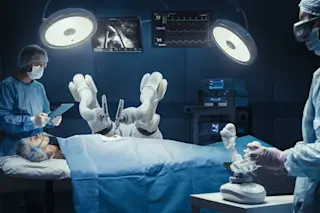
Just outside Washington, D.C., in a pink metal shed owned by the Smithsonian Institution, upstairs in the conservator's lab, stretched out on a table, lies Harrison (Jack) Schmitt, looking slightly crumpled. Actually it's his empty suit that lies here, still filthy with moondust. It's an ideal museum artifact, really, because it transports you immediately from one time and place— December 2000, Suitland, Maryland— to another: December 1972, Taurus Mountains, the moon. But there is also something sad about Schmitt's suit, and it's not just that he and Gene Cernan of Apollo 17 were the last men to walk on the moon. Schmitt's neoprene-latex gloves are growing brittle with age— though not as brittle as Neil Armstrong's, which would crumble if you squeezed them. If you could peer through the hale exterior of Teflon-coated fiberglass, you would find that the multiple layers of Mylar that once protected Schmitt from the lunar elements are now themselves in doubtful condition. And the long underwear— you don't even want to look at it. All that PVC tubing is sure to be a mess.
We modern folk have a peculiar problem. No culture before ours has been as concerned with understanding the past (archaeology was only invented in the 19th century) or with transmitting an understanding of itself to the future (time capsules). No culture has been as conscious of its place in history, as compulsive about preserving artifacts of its own age. At the Smithsonian museums in Washington you can see not only space suits but also Barbies, Hula-Hoops, and bicycle helmets. But our artifacts mock this attachment. They are not made of stone or ceramic or even, for the most part, of metal. We live in the Age of Plastic— and plastic does not last.
"Plastics transformed our culture," says Mary Baker, a polymer chemist who worked for the Smithsonian until recently and now advises various museums. "People have more things in their lives now because of plastics. If we had to make everything out of stone or metal, only rich people would have them. The catch is, because plastics are so easily made, they are easy to degrade."Ironically, plastics are often condemned for not being degradable enough and for persisting too long in the environment once we've thrown them away. But when you start to think of plastics as museum curators do— as artifacts of our civilization rather than as trash— they appear anything but long-lived. Plastics and their kin, synthetic fabrics, are made of polymers: long-chain molecules made of repeating links known as monomers. Polymers are all subject to attack by heat or light, moisture or oxygen, acid or base, and are all destined to fall apart, each in its own way and in its own time. Often no external attack is needed.
Take cellulose nitrate, the first plastic, invented in the mid-19th century and also known as celluloid or nitrocellulose. It's basically a chain of sugar molecules with molecules of nitrate, or NO2, hanging from various points of the chain. The plastic was once widely used to make film, even though it had the unhappy habit of bursting into flame in movie projection rooms. But that's not the problem that worries museum curators today. Pure cellulose nitrate is brittle, and to make it malleable, chemists had to add a so-called plasticizer— usually camphor, the same stuff that used to give mothballs their smell. Over time, unfortunately, the camphor escapes from the plastic and into the air, restoring the nitrocellulose to its old brittle self.
Cellulose acetate, which replaced cellulose nitrate, is fire resistant and not brittle, so it doesn't need a plasticizer. But that doesn't mean it's stable. When cellulose reacts with acetic acid to form the material, some acetic acid isn't used up. It gets trapped in the plastic— and slowly starts to eat its way out. "It's basically toasting the cellulose," Baker says. The plastic turns amber, loses mass, and starts to shrink. Curators start smelling vinegar— of which acetic acid is the aromatic ingredient— and the film or other artifact disintegrates, often spectacularly. "There is all this stress under the surface," explains Baker.
One of the biggest stresses for curators these days is polyvinyl chloride, or PVC, also known as vinyl. It is a very simple polymer— just a long carbon backbone carrying hydrogen and chlorine atoms— and everything from Barbie dolls and countless other toys to LP records to domestic drainpipes are made of it. (The little tubes sewn into the astronauts' spandex long johns to channel cooling water over their skin were made of PVC.) The main problem with PVC, as with nitrocellulose, is its natural brittleness. In most applications (though fortunately not in pipes) it has to be softened by mixing in a compound called a phthalate ester.
Like the camphor in nitrocellulose, phthalate will come out— and sometimes, says chemist Yvonne Shashoua of the National Museum of Denmark, it migrates to the surface of the plastic surprisingly fast. That process has recently become a public health concern, because of fears that phthalates, including the common one known as DINP, could mimic the effects of estrogen, the female sex hormone, thus potentially damaging the reproductive system. The European Union has banned the use of phthalates in food containers and toys that small children might put in their mouths. The museum curator's concern, on the other hand, is that Barbie and other PVC artifacts could lose their sex appeal and even their structural integrity in less time than it takes for the present to become the past. "The problem is that these plastics are only designed to last for five or 10 years and then be replaced," says Shashoua. "But museums can't take that attitude." She has found that some PVC artifacts get sticky with exuded phthalate in less than 15 years.
For better or worse, some modern plastics, with their complex engineering, may turn out to be less ephemeral. For instance, acrylonitrile butadiene styrene, a.k.a. ABS, is strong and tough enough to use in car parts. Another plastic that appears to have better-than-average prospects is polyethylene terephthalate, or PET, the stuff of soda bottles and polyester suits, among a thousand other things. Old films made of cellulose nitrate or acetate are now being copied onto films made of PET. It is so durable and chemically inert that Baker recommends PET jars for use as family time capsules, but not if you have the time horizons of an Egyptian pharaoh. "I feel pretty confident about saying 50 years," she says, speaking generally about the life expectancy of the best plastics. "A hundred years? That's enough time for a problem we haven't noticed yet to crop up."
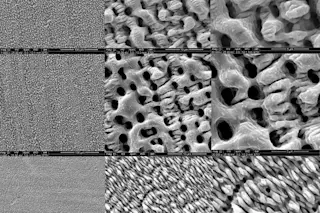
Some parts of the Apollo suits may not make it even that long, notably the gloves and the joints, which were made of synthetic and natural rubbers. The astronauts' vigorous movements on the moon created cracks in the rubber, sweat and oxygen got into the cracks, and the rubber hasn't responded well. In some places the oxygen has caused the chains of the isoprene polymer to break down into shorter molecules with fewer cross-links between them, making the rubber more fluid; in other places, often in the same joint, more cross-links have formed and the material has gotten brittle and crumbly. "Those parts you might as well wave good-bye to," says Baker. The boot linings of John Glenn's Mercury space suit, a few years older than the Apollo suits, have already crumbled and gone. They will survive only in documents.
But Baker, for one, is optimistic about our chances of somehow transmitting the plastic essence of our culture to our distant descendants— if only because we try so hard. For a while, she says, plastics had to struggle for respect in the curatorial world. ("If you're trying to grab part of a limited museum budget, it's a whole lot easier if you have the Mona Lisa than if you say, 'Well, I'd like to preserve this transistor radio.' ") But that is changing. At the Smithsonian facility in Suitland, conservator Lisa Young has a two-year "Save America's Treasures" grant to study the Apollo suits in minute detail and figure out how best to store and handle them. She will be CT-scanning each and every suit to monitor its internal state of decay. "I hope they will be around for at least a hundred years," she says.
The Egyptians had better material to work with, but they weren't trying to build time capsules; they were just trying to give the pharaoh a comfortable ride in the afterlife. Baker has no doubt that the museum's efforts are worth it. "We don't want people to say some day, 'Maybe space aliens taught them how to do this,' " she says, meaning Barbie and the moon landings and the whole plastic tapestry that is us. "If people had said that to the makers of the pyramids, maybe they would have documented their work a little better."