Did we talk about my pine tree?” says Drew Endy. I nod. I have heard his pine tree spiel (and you will, too). So he turns to another example to make his point: a plastic garbage can near his office door. His plan is simple: fill up the can with sawdust, add some “programmed wood fungus,” as he calls it, and leave.
“I come back a week later, and I shake out all the extra loose sawdust and spent materials, and out comes my new laptop,” he says.
“Are you serious?” I blurt out. I must sound incredulous, and I am.
“I’m serious,” he says, sitting cross-legged on his swivel chair.
I have caught Endy, an associate professor of bioengineering at Stanford University in California, at a cusp in his career. A boyish-looking 40-something, he has spent the past decade trying to reimagine the very idea of manufacturing by co-opting the inherent intelligence and capabilities of biology — partnering with biology to change how we make things, how we live. It has meant ignoring naysayers and believing in a vision that is as difficult to comprehend as it would have been to imagine an Apple iPad in the days of the first mechanical computer.
But persistence seems to be paying off. A slew of recent successes means that what seemed a pipe dream to many is looking much less so, especially to Endy and a growing band of believers. “It is a moment in time where there is a lot of burden relieved and a lot of freedom and dancing,” he says.
At the heart of their vision is a form of bioengineering called synthetic biology, which would let them rewire biology to make organisms do our bidding — from making drugs, biofuels and fragrances to the decidedly futuristic manufacturing of laptops from sawdust. It’s a grand vision for a new way of being: in tune with biology, rather than destroying it.
Biology is an unusual ally for Endy, who trained as a civil engineer and worked with reinforced concrete, sewage treatment and even managed construction one summer for Amtrak. “Biology is a manufacturing partner that is the most amazing I have ever encountered,” he says.
"There is this natural technology out there in the wild that is so capable of manufacturing stuff that it coats the surface of the Earth. It takes atoms from the atmosphere and light from the environment and self-assembles huge structures with atomic precision."
To make his point, he once asked his students, all sophomores taking his bioengineering laboratory class at Stanford, to look out the classroom window and point out the things that impressed them. Some picked out the 150-foot-wide radio telescope in the Stanford foothills. Others were impressed by the new quarter-billion-dollar bioengineering building.
No one picked out what Endy was eyeing — the green, forested coastal ridgeline. “What impresses me is that ridgeline, and not just the fact that there is a ridgeline, but that it’s covered with this wild biology,” he says. “There is this natural technology out there in the wild that is so capable of manufacturing stuff that it coats the surface of the Earth. It takes atoms from the atmosphere and light from the environment and self-assembles huge structures with atomic precision.”
Precise Biology
It’s this atomic precision that enamored Tom Knight, often called the godfather of synthetic biology. In the 1980s, Knight was at MIT designing very large-scale integrated circuits (VLSI) out of silicon, the basis for electronic computing. But his crystal ball was getting cloudy. He could see that Moore’s Law, which observes that the number of transistors crammed into integrated circuits doubles every two years, would eventually plateau, “which is what is happening right now,” says Knight.
That’s because of technological limitations. Semiconductors are made by bombarding pure silicon with atoms of phosphorus or boron, thus “doping” silicon to turn it into a semiconductor.
The process has inherent randomness. As transistors get smaller and smaller, occupying ever-tinier regions of a silicon chip, it becomes increasingly likely that any given region (barely tens of nanometers across) may have too many or too few dopant atoms.
“You are losing control over the fabrication of those devices, and you could see that coming,” says Knight. “I decided the future was going to be based on a technology where you get to put atoms where you want them.”
That technology is all around us, and it’s called biology. At its core, the idea behind synthetic biology is simple: design and build biological systems to do what we want them to do. To do that, you need to design DNA sequences and insert them into the bacterial cells of your choice, a technique pioneered by the first generation of genetic engineers. But the early days, the late 1990s and earlier, were rough. Everyone had their own way of making snippets of DNA. “That drove me crazy as an engineer,” says Knight.
He wasn’t the only one feeling frustrated. Fast-forward to the beginning of the new millennium. Endy, who had by now retooled himself as a bioengineer, was at MIT, too. Knight, Endy and many others — the list of participants reads like a who’s who of today’s synthetic biologists — organized meetings and workshops in Massachusetts, Virginia and California. They asked themselves: How do we get much better at engineering biology?
The answer was threefold. First, synthetic biology needed standardized parts, much like how computer engineers design and build using standard electronic components with well-defined interfaces. Or like the nuts and bolts of mechanical engineering. To Endy, the notion of standards goes back to the Roman Empire. Take the aqueduct of Segovia, Spain. “You’ll see that it’s not made from rocks that were lying about the countryside,” he says. “It’s made from standard rocks, and the standardization allowed for coordination of labor, over location and across time.”
The second part was the need for abstraction, a concept that is abundantly clear to, say, computer programmers. High-level programming languages, like Java or C++, provide access to the underlying hardware via layers of abstraction, so you can write data to a file on a disk regardless of whether the disk is a magnetic hard drive or flash memory. Synthetic biology had to embrace abstraction so that designers could make a genetic program without having to memorize and fiddle about with every specific sequence of DNA.
The third, related requirement was to separate designers from builders. Think architecture and construction: The architect draws up the plans and hands them over to the building contractor. The synthetic biologists argued that they needed to embrace this, too.
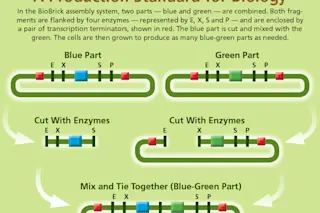
To this end, Knight invented the first BioBrick standard for assembling DNA parts. The resulting parts are pieces of DNA with specific ends that act as targets for enzymes that cut and splice DNA. This allowed other researchers to mix and match BioBrick parts via a standard construction process, and then insert the composite parts into cells to make them carry out a designed genetic program.
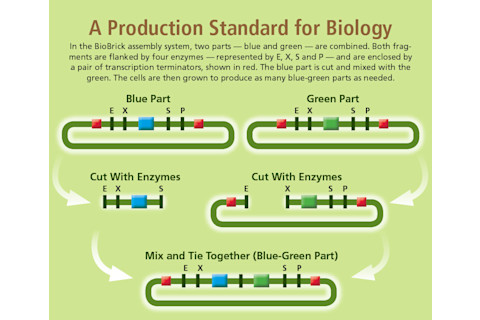
Dan Bishop/Discover
In 2004, these early principles were tried out by students during a synthetic biology competition, now known as the International Genetically Engineered Machines (iGEM) contest, an effort that grew out of Endy’s and Knight’s labs. Teams from five U.S. universities vied to build a biological system using standardized parts. In 2013, more than 200 undergraduate teams from around the world competed in iGEM, creating novel biological systems (such as an E. coli-based sensor to detect arsenic in drinking water) using BioBrick parts made and freely shared by previous iGEM students, and stored and distributed from the freezers at iGEM headquarters in Cambridge, Mass.
Despite the dramatic growth in such student-led efforts, many still doubted whether the principles of standardization and abstraction were applicable to biology. Endy recalls a 2006 New York Times article on synthetic biology in which Frances Arnold, a distinguished professor of chemical engineering at Caltech, said, “There is no such thing as a standard [biological] component, because even a standard component works differently depending on the environment. … The expectation that you can type in a [DNA] sequence and can predict what a [genetic] circuit will do is far from reality and always will be.”
Endy paid heed to such criticism, and it weighed on him. “I have been carrying a technical, scientific burden,” he says. “It’s hard to ignore when smart people say what you are trying to do is impossible.”
Engineering a Transistor
As far as Endy is concerned, that burden has lifted somewhat in the past two years. In June 2012, his Stanford lab published what would turn out to be the first of three key papers that advanced the cause of computing inside cells using standardized parts. In the first paper, a team led by postdoc Jerome Bonnet showed how information could be stored inside a cell. Granted it was just a single bit of memory, but there it was: the ability to store either a 0 or 1, and flip the bit at will.
To do this, the team borrowed know-how perfected by bacteriophages — viruses that can infect bacteria by inserting their viral genome into the bacterial genome. At the heart of the “bit” of memory is essentially a fragment of DNA called a promoter, which enables the machinery of a cell to make a given protein. In this case, if the promoter faced one way (0), it would enable the production of a green fluorescent protein inside E. colicells. If the promoter were facing the other way (1), a red fluorescent protein would be produced.
The team showed how they could flip the direction of this promoter using bacteriophage enzymes, whose levels inside the cells they controlled using chemicals. Once flipped, the DNA sequence would continue to be replicated by the bacteria, thus maintaining the bit as 0 or 1 until it was flipped again. It had been a long, draining effort to get this simple element working. “It took three years to get a single bit of memory out of the lab,” says Bonnet.
Later that year, the team built another cog in the wheel of biological computing: the ability to send arbitrary messages between cells, again with help from a bacteriophage called M13. This wondrous virus can package its DNA into “particles” and send them to other cells without killing the host cell. Endy and his student Monica Ortiz tweaked M13 to send arbitrary DNA messages of their own choosing, instead of viral DNA, from one cell to another.
But the singular achievement in Endy’s eyes came in 2013, when his team built its first biological amplifying logic gate. In conventional digital electronics, the key to building logic gates is the transistor, a component in which a modest control signal can regulate the flow of a large electric current. Their biological transistor, which they named the “transcriptor,” is a device made from an engineered sequence of DNA. The “current” is the flow of a molecule called RNA polymerase, which moves along the DNA “wire” and transcribes it, allowing the cell to make the relevant protein. The key here was to create a control signal that could let the RNA polymerase transcribe or stop it from doing so.
To do this, they embedded within the DNA strand another bit of DNA called a transcription terminator. They then used bacteriophage enzymes to flip the direction of this terminator — technology perfected while creating the 1-bit memory. In one direction, the terminator allowed the RNA polymerase to zip across the DNA sequence and transcribe downstream genes. Flipped, it prevented transcription. Endy and his team had their basic biological transistor, and they used it to implement a variety of Boolean logic gates, such as AND and OR.

San Francisco artist Phil Ross uses wood fungus to create super-strong bricks. | Courtesy Phil Ross, 2013
To Endy, the team’s paper in Science detailing these logic gates did not tell the full story. “What was mind-boggling to us was that every one of our DNA designs worked the first time,” says Endy. “We didn’t have to go around the design-build-test cycle multiple times.” Standardization and abstraction had finally worked — so much so that the parts they created are now freely available and being used by others to implement genetic computing in a variety of organisms.
While performing this work, Endy was involved with BIOFAB, a facility in Emeryville, Calif., that he co-founded with bioengineer Adam Arkin of the University of California, Berkeley. By 2013, BIOFAB had produced a first collection of reliably reusable DNA parts for synthetic biology. Among the key elements engineers need to get an organism such as E. coli to make a protein from a synthesized gene are extra sequences such as promoters (to help the cell make RNA from DNA) and ribosome binding sites (or RBS, which the cell needs to make proteins from the RNA).
Over the years, researchers have identified a plethora of promoters and RBSs that work in E. coli. “But their performance in combination is not reliable and predictable,” says BIOFAB’s Vivek Mutalik, a synthetic biologist who was lured by Arkin and Endy to work on the project. “There was a 50 percent chance that things will work for one gene and not work with another gene.”
Mutalik led the effort to design a set of promoters and RBSs that could be attached to any “gene of interest” such that a bioengineer could be near certain (well, 93 percent sure, but that’s near certain in biology) that the protein could be made within an organism to a desired target range. The team tested various combinations of promoters and RBSs and characterized their performance. In essence, they were able to assign to each part a “quality score” that tells a designer how effective the part will be in a given context. These parts, too, are now freely available for public use and were instrumental in getting Endy’s logic gates on the first try.
Taken together, these developments have lightened the load on Endy’s shoulders. “Psychologically, if you will, or operationally, you are catching me at this moment between the last decade and the next decade. When posed with the question, ‘Can we make biology easy to engineer?’ the answer is, ‘Yes, it’s not impossible.’ It wasn’t obvious the answer was going to be yes,” he says. “In a very real way, I am liberated.”
Pine Power
That’s probably why he’s letting himself dream, which brings us back to the pine tree, the one in the front yard of his home in Menlo Park. For two years now, Endy has been watching the tree shed hundreds of pinecones. “I finally couldn’t escape from the fact that there is a surplus manufacturing capacity in my front yard,” says Endy.
The tree was turning undifferentiated balls of sap into elaborately structured pinecones. He figured that the 32,000 people of Menlo Park each threw away about 500 pounds of garden waste each year. That’s 16 million pounds — far greater than the worldwide production of engineered silicon to make computer chips. Could a pine tree be used to produce computer chips? “There is no chance today that I can make my pine tree make computer chips rather than the pinecones,” says Endy. “But if I got really good at engineering pattern formation within living systems, change the fertilizer a little bit, make connections between the organic and the inorganic, there is a chance that I could create a new kind of cash crop in my front yard.”
If you leave aside the pine tree and the sawdust-eating, laptop-producing wood fungus for now, there are humbler attempts that are showing us the way. San Francisco artist Phil Ross has already begun taking baby steps toward the kind of future Endy envisions. He has used the biological might of wood fungus, primarilyGanoderma lucidum, to make bricks that could one day lead to buildings.
He begins by stuffing sawdust into airtight bags and steam-cooking them. He waits for the bags to cool before adding fungal cells, which begin to transform the sawdust. While the mix is still squishy, Ross puts each bag into a mold and waits. Eventually, the fungus consumes the sawdust. “It can do a complete biotransformation,” says Ross. “It can turn all of that wood completely into its own body.”

Phill Ross' arch, built with wood fungus bricks, was part of an exhibit in Germany. | Courtesy Phil Ross, 2013
That body, it turns out, is a rigid structure of the biopolymer chitin. Each brick ended up having a spongy core but a tough outer skin so hard that the tools used to try to cut and shape the bricks were ruined. So molding the bricks in the right shape in the first place helped. Ross’ team showed how the bricks could be stacked to build an arch in a museum in Germany. At the end of the exhibit, pieces of the bricks were mixed in hot water and served as herbal tea — a demonstration of the biodegradability of the material. Now he’s working on growing furniture, much of which is on display in San Francisco.
How far can we push such ideas? “It’ll transform the world,” says Ross. “It’ll create material wealth that will make our current standards of materiality seem trivial and obtuse.” As with my earlier encounter with Endy’s vision, I’m taken aback.
“You are saying that seriously, not facetiously?” I ask.
“I’m absolutely being serious,” he replies. “What if you can imagine that there was as much of anything that you could possibly want. Rather than having to hold on to things because of a perceived future value, if you just grew things according to your want and desire, and it cost almost nothing, and it took almost no energy, and it was fully biodegradable — our ideas of material reality would go through a gigantic transformation.”
To go from bricks to laptops would mean engineering the wood fungus to do exactly what we want it to with atomic precision, using potentially the kinds of reusable biological parts being designed in Endy’s lab and elsewhere. But where is this going in the near term? “Don’t expect miracles in the next five years, but watch out 10 years from now,” says Knight. But predicting exactly what synthetic biology will enable even 10 years hence is like trying to predict cell phones when vacuum tubes were invented.
Ethics of Building Sequences
Given all that, isn’t anyone worried about how far this could go, and whether things could go badly wrong? “We are all worried,” says Mukund Thattai of the National Centre for Biological Sciences in Bangalore, India. Thattai was at MIT when iGEM was launched, and he has helped judge iGEM entries and mentored students on their projects.
One such project arose out of ethical concerns raised by artists in Bangalore. The project imagined a future, 100 years from now, in which synthetic biology was the norm and BioBrick parts were ubiquitous in organisms around us. How would we know whether humans had introduced those DNA sequences unique to BioBrick parts? The students went around Bangalore and into the forests of the state of Karnataka, brought soil samples and looked for BioBrick-specific DNA sequences in the soil microbes. They found nothing. “So if you ever find them, 10 years from now, 100 years from now, they came from human interference,” says Thattai. “We should be aware of what we are doing. We should engage with the public, we should engage with artists, we should think about the ethics of it. But we should accept that this is coming.”
Endy accepts — embraces, really — the idea of a synthetic biological future. Not doing so, and continuing to live and manufacture and consume the way we do, is not an option for him. “We are destroying environments, we are critically ripping away biodiversity; it’s a disaster,” he says. Partnering with biology — ethical concerns and obvious dangers notwithstanding — could help transform us. “We actually have a chance of reinventing civilization,” he says.
[This article originally appeared in print as "Nature's Technician."]