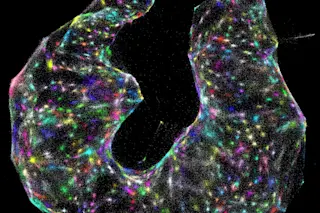
Joshua Weinstein spent hours in graduate school pulverizing zebrafish in a blender. That was a normal first step toward sequencing the fish’s genes, but it had a high “ick” factor — and he felt like it wasn’t very good science. Although his results would tell him about the fish’s overall genetic activity, they couldn’t reveal which genes were present and active in different places: like inside the fish’s immune tissue or its nerves, or within a cancerous tumor. And he wanted to know.
Joshua Weinstein (Credit: Jean Lachat/University of Chicago)
Jean Lachat/University of Chicago
So Weinstein co-developed, and this year unveiled, what he calls a DNA microscope. It’s actually not a physical piece of lab equipment, but a technique that allows researchers to examine precisely what genetic activity is happening where. Although some colleagues quibble with calling the development a microscope, several agree that it could offer profound new insights ...