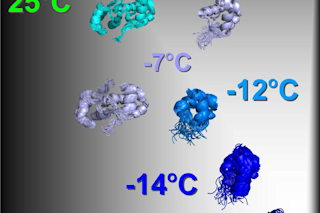
“Snapshot” of the unfolding of CylR2 protein from Enterococcus faecalis. If the protein is cooled from 25°C to -16°C, it successively breaks down into its two identical subunits. The latter are initially stable, but at -16°C they form an instable, dynamic protein form, which plays a key role in folding. © Zweckstetter, Max Planck Institute for Biophysical Chemistry & German Center for Neurodegenerative Diseases Proteins — tools of living cells — can’t do their job if they’re not in shape. Literally. And a new study is the first to image the various stages of a protein's undoing, which will lend valuable insight to treatment of diseases such as Alzheimer’s and Parkinson’s. Those are just two of the diseases caused by proteins that are misfolded — their amino acid chains are not arranged correctly, resulting in a misshapen three-dimensional structure. When misfolded, these proteins don’t work and, in the case of ...
Protein "Filmed" Unfolding for the First Time
Discover how CylR2 protein unfolding reveals insights into misfolded proteins and their role in diseases like Alzheimer’s.
More on Discover
Stay Curious
SubscribeTo The Magazine
Save up to 40% off the cover price when you subscribe to Discover magazine.
Subscribe