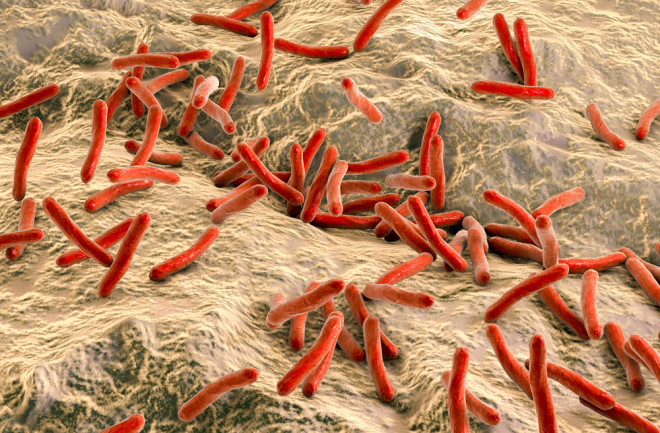
For the past 25 years, Anura Rambukkana has been studying a disease that’s already been cured. He studies leprosy, a disease that was once the scourge of humanity before a course of drugs developed in the mid-20th century brought it under control.
For decades, he’s worked in a field that sees little funding and few new faces, and many of his contemporaries have moved on to higher-profile projects involving diseases that attract grant dollars. Rambukkana, a professor of regeneration biology at the Centre for Regenerative Medicine at the University of Edinburgh, likely would have joined them, but for a singular captivation by the leprosy bacterium — and the hunch that it might have something to teach us.
Anura Rambukkana. (Credit: University of Edinburgh)
Working as a graduate student in Amsterdam analyzing skin samples from leprosy patients, he picked up on an intriguing facet of the bacteria’s behavior. The cells it infected didn’t die — in fact, they looked exceedingly normal. It was a hint that the bacteria had ulterior motives.