Scientists at Rockefeller University claim they’ve pinpointed a protein in the ear that acts as a sort of molecular gatekeeper, helping convert soundwaves into the electrical signals that our brains interpret as sound. The finding, though incremental, helps establish a more detailed understanding of how hearing works.
Inner Ear Discovery Helps Explain How Sound Waves Become Brain Signals
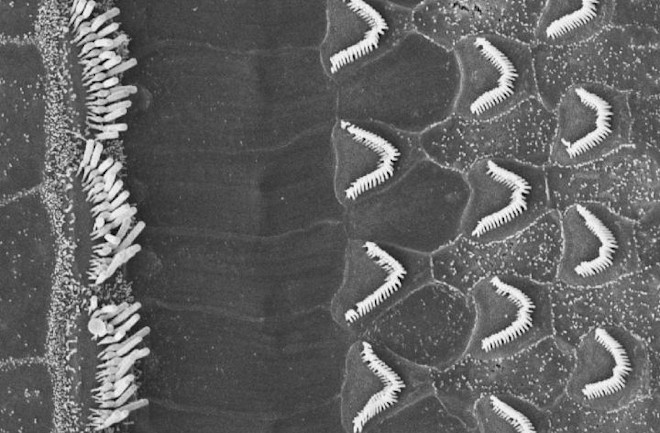
A high-powered microscope shows mouse ear close up, revealing bundles of inner and outer hair cells in the cochlea. The white filaments here are hairs of the bundles. Those are connected by tip links.(Credit: Tobias Bartsch)
Newsletter
Sign up for our email newsletter for the latest science news
0 free articles left
Want More? Get unlimited access for as low as $1.99/month
Stay Curious
Sign up for our weekly newsletter and unlock one more article for free.
View our Privacy Policy
Want more?
Keep reading for as low as $1.99!
Already a subscriber?
Find my Subscription
More From Discover
Stay Curious
Subscribe
To The Magazine
Save up to 40% off the cover price when you subscribe to Discover magazine.
Copyright © 2025 LabX Media Group
