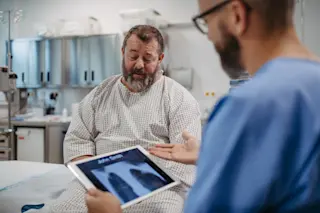
“He was locked in the bathroom,” said the young woman.
“In the bathroom?” I echoed skeptically. “How’d he get there?”
“Last week he wasn’t feeling well and had trouble breathing. He didn’t come in Friday. I called over the weekend. No answer. He lives alone. When he didn’t come in today, I called the police.”
The cop at her side chimed in. “We had to break in. He wouldn’t come out.”
“Are you a relative?” I asked the young woman.
“No, just a friend. Will he be OK?”
“I expect so. Do you know his medical history? Any psychiatric problems? Drug use?”
“No. He’s a sweet, thoughtful person. Very smart. He manages our accounts.”
Throughout this exchange, my patient, a solidly built 30-year-old, sat quietly on the stretcher. I went to him.
“How are you, sir?”
“OK.” He stared straight ahead, looking more lost in thought than confused.
“Why were ...