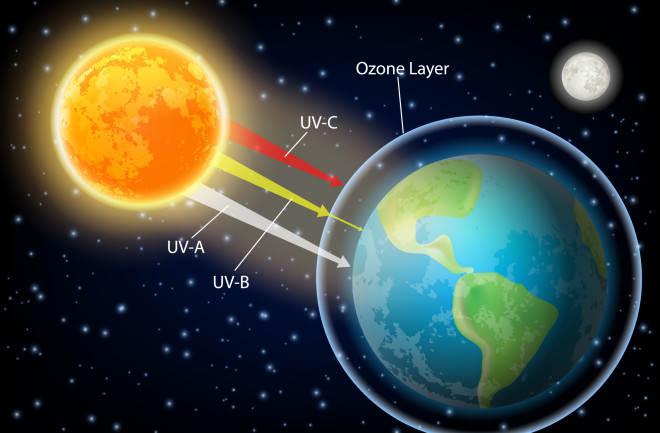
Almost 40 years ago, scientists discovered that human activities were depleting the ozone layer and that a hole had formed over Antarctica. These sobering discoveries brought the world together to tackle an environmental challenge of a lifetime.
Decades later, the ozone hole has come a long way since its dire outlook in the 1980s. But it's not a cause for celebration just yet. There are still ongoing efforts between policymakers and scientists to ensure the ozone in the atmosphere is protected. In light of the hole’s ongoing recovery, here’s a guide to how the hole formed, the coordinated effort to fix it, and whether it's likely to ever to take a turn for the worse.